
The results of a level I clinical study featuring Attrax Putty, our next generation surface optimized ceramic, are now available from Spine. This multi-center, intra-patient, randomized controlled trial investigated the efficacy of Attrax Putty as a bone graft substitute for autograft in instrumented posterolateral thoracolumbar spinal fusions. For each patient, Attrax Putty was randomized to one side of the posterolateral spine and autograft was placed on the opposite side. The major advantage of this study design was the elimination of interpatient variability. Upon assessing fusion by CT scans at one year, the study concluded that the fusion performance of Attrax Putty alone was non-inferior to autograft in instrumented PLF procedures.1
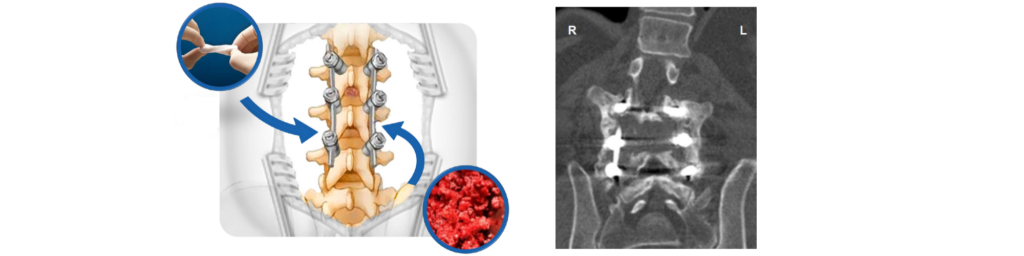
Attrax Putty is now the first and only ceramic supported by Level I evidence as a bone graft substitute in PLF.1,2 Previously, other ceramics were only FDA-cleared as bone graft extenders, which require the addition of the patient’s own bone to be effective. This RCT answers the call from surgeons and hospitals for higher quality data on which to base clinical and purchasing decisions. The publication of these results further reinforces NuVasive’s position as a leader in the spine market, offering innovative solutions to support positive clinical outcomes.
Attrax Putty is a synthetic, bioactive and osteoconductive bone void filler for the repair of bone defects. This proprietary, advanced biomaterial features a surface microarchitecture which provides an instructive environment for bone formation without added cells or growth factors. The moldable graft material is resorbed and replaced by bone during the healing process.
Click here to learn more about Attrax!
For complete important product safety information, refer to the Attrax IFU found at nuvasive.com/eifu.
1 Lehr MA, Oner CF, Delawi D, et al. Efficacy of a standalone microporous ceramic vs. autograft in instrumented posterolateral spinal fusion; a multicenter, randomized, intra-patient controlled, non-inferiority trial. Spine 2020;published ahead of print.
2 Based on review of publicly available materials at the time of this release.
#9512911 Rev A