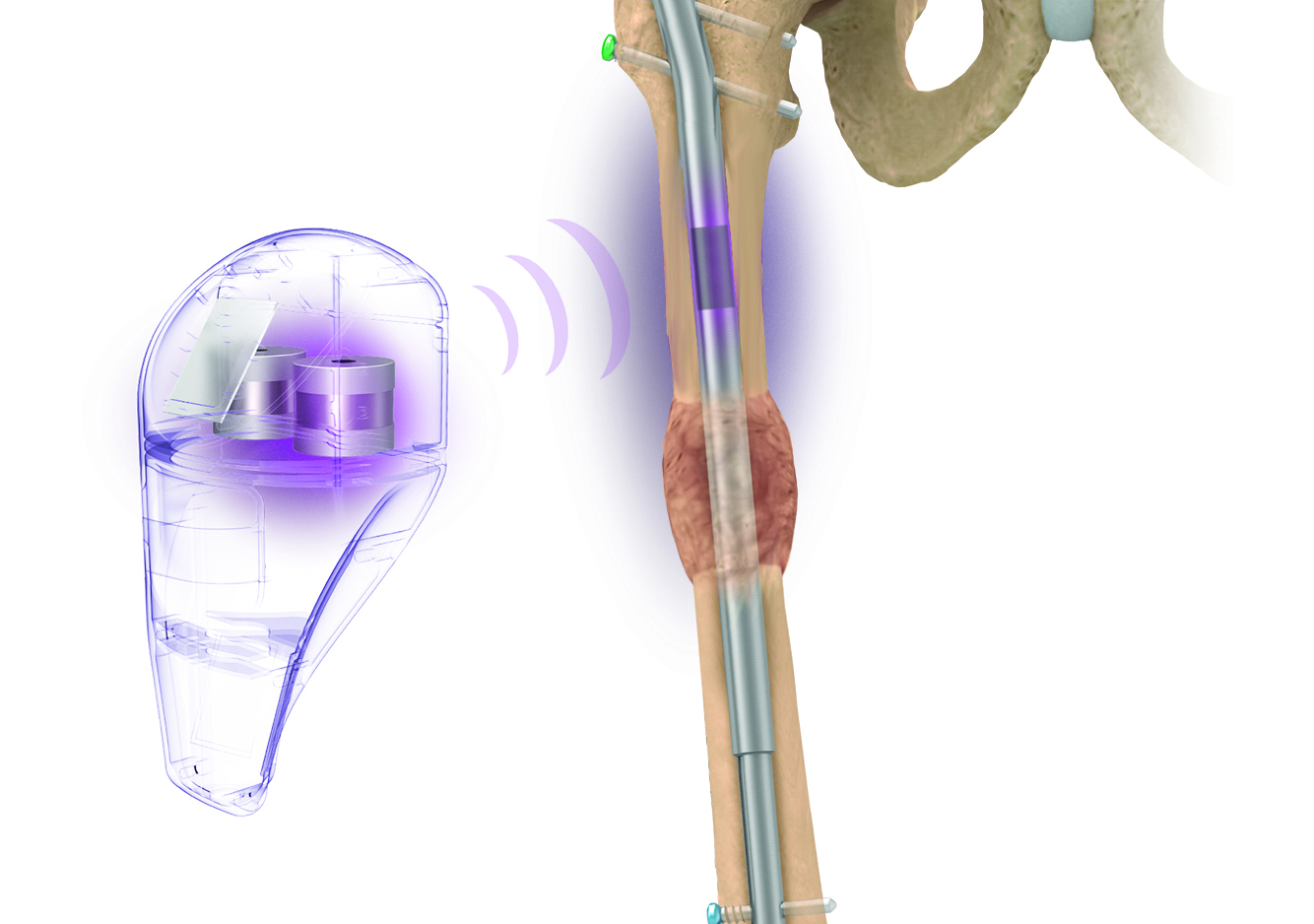
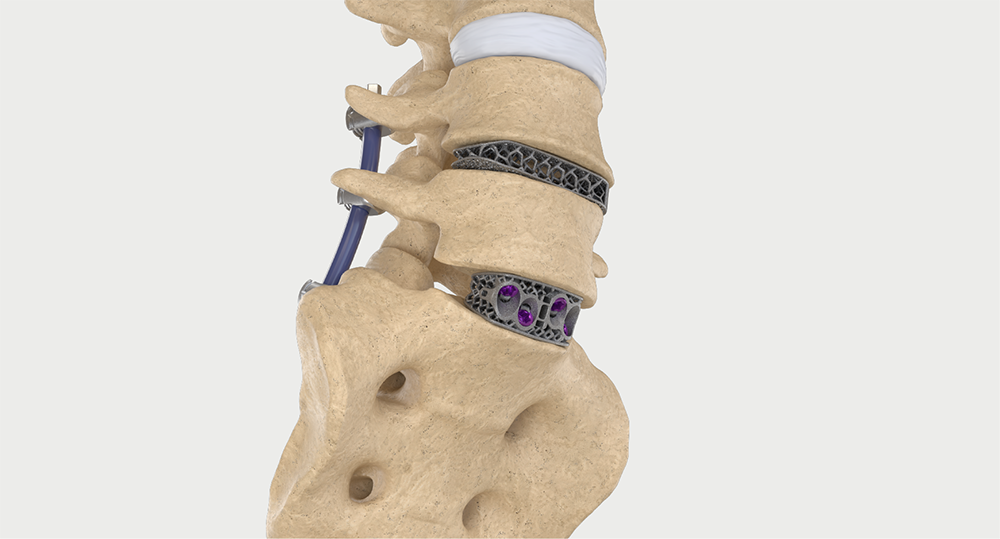
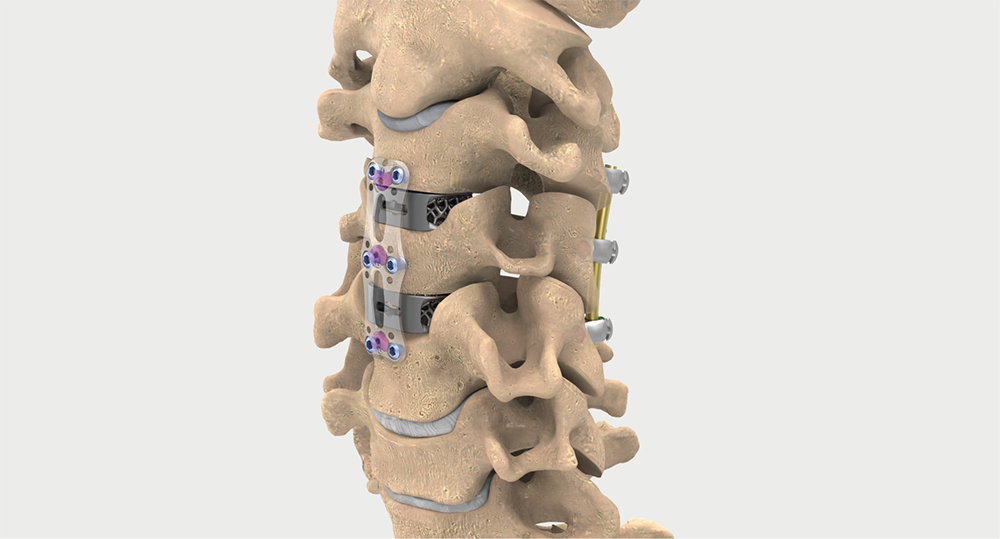
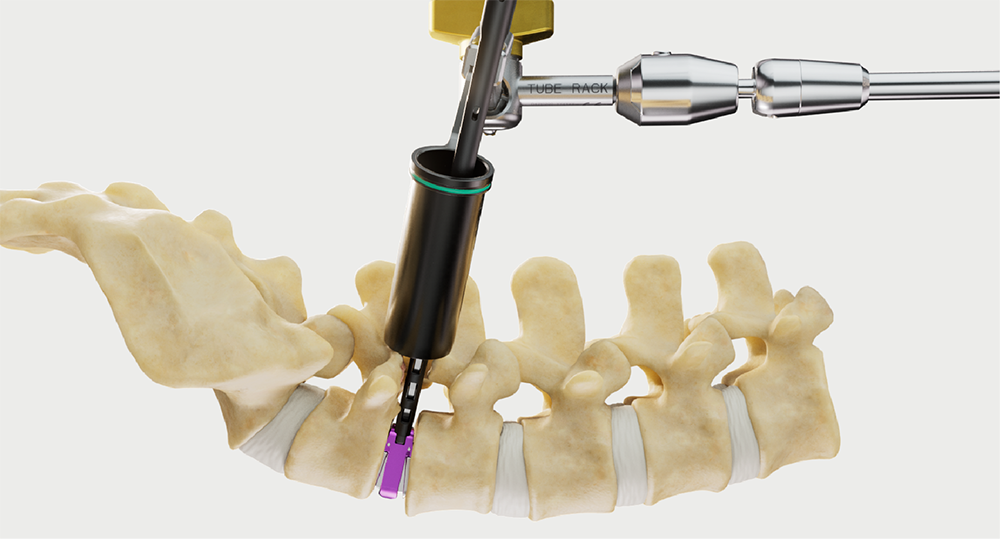
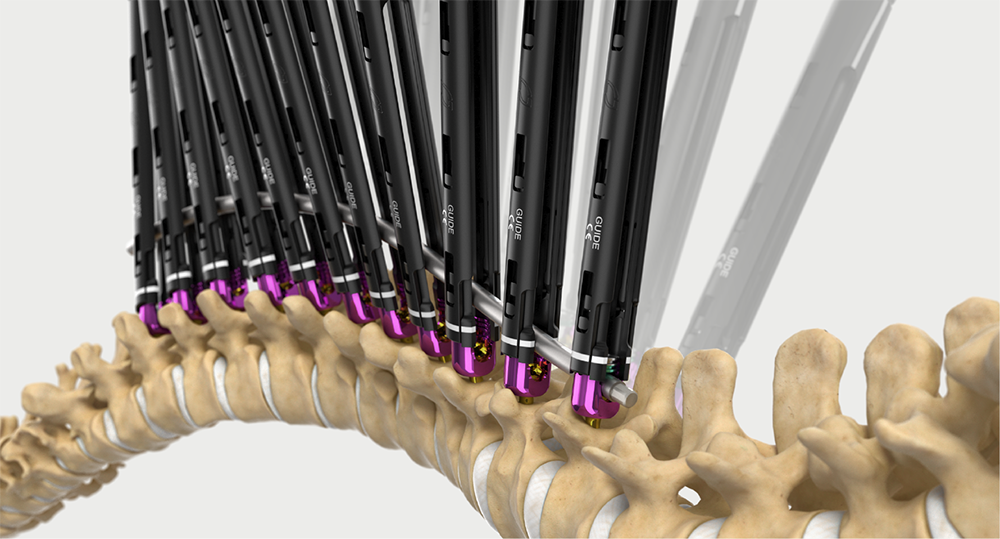
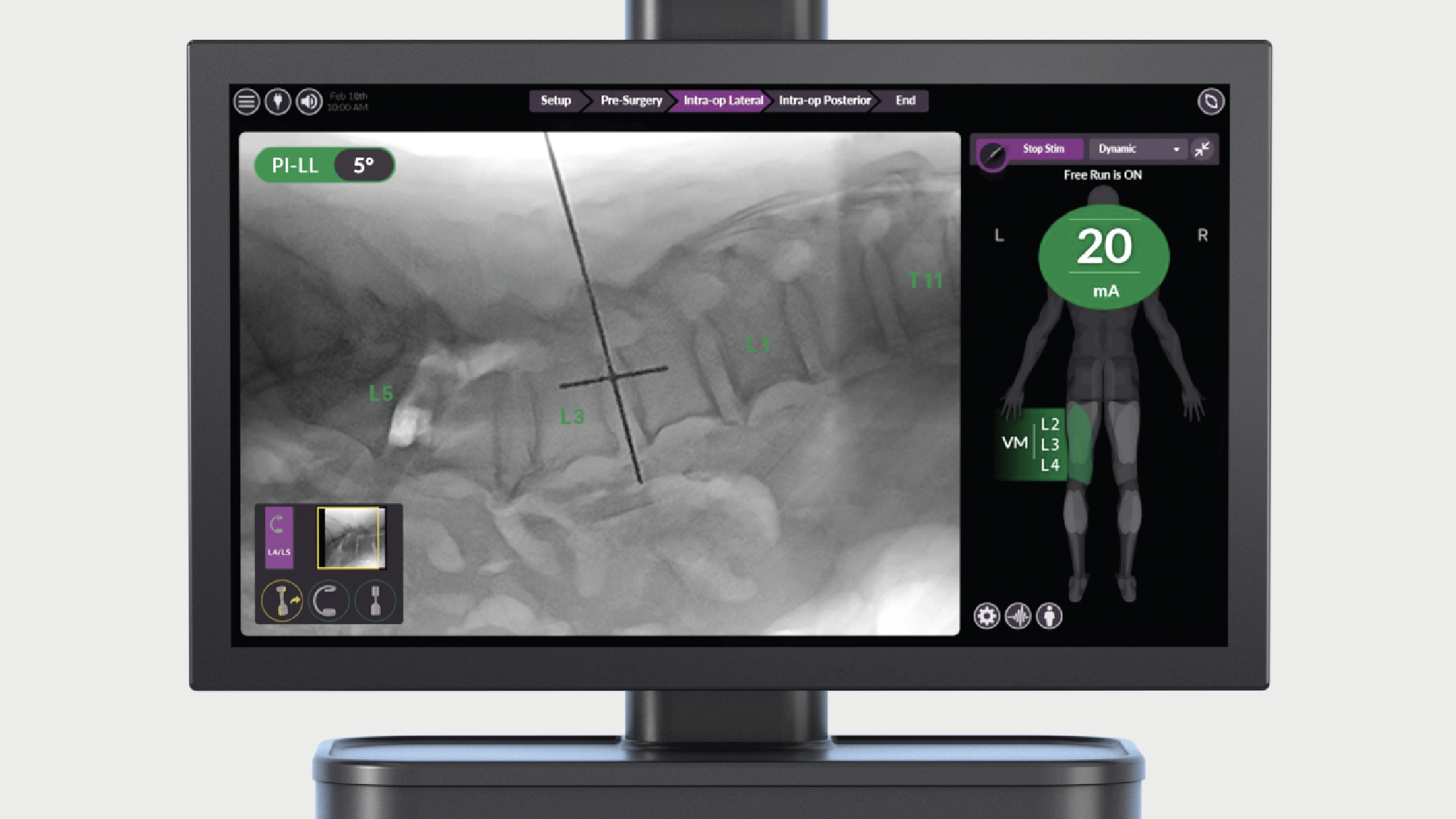
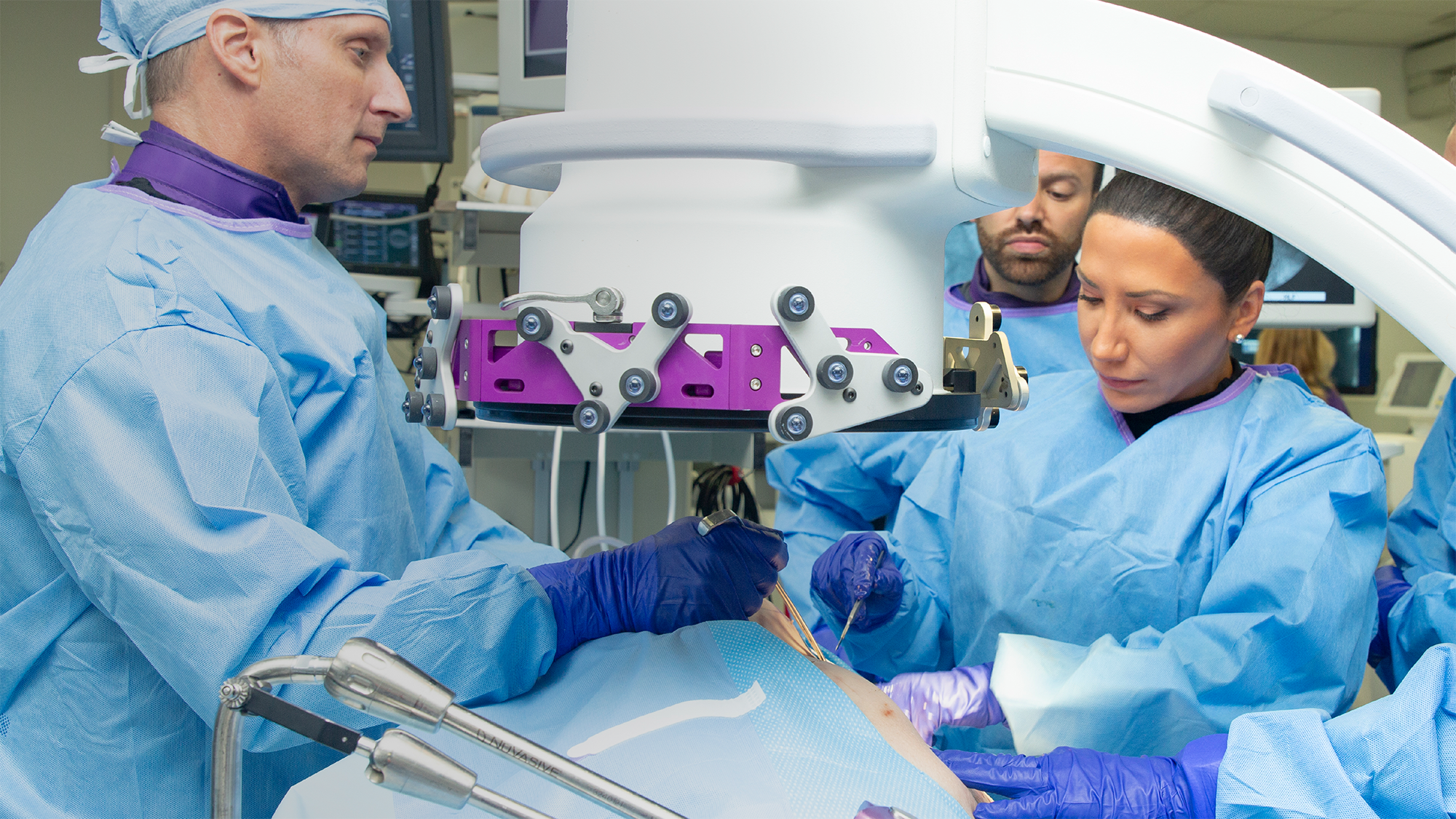
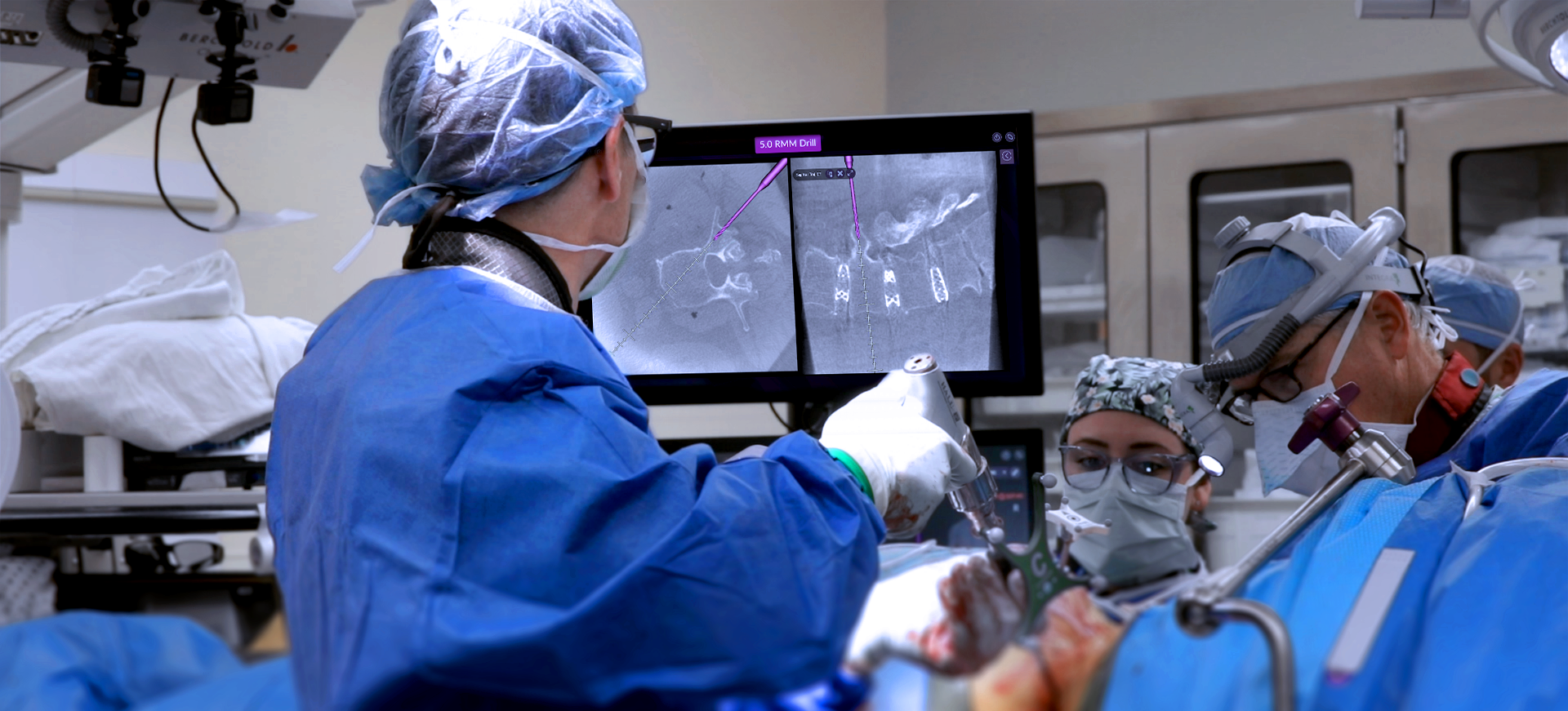
Intelligent surgery is the next evolution of our innovation
Outcome-driven innovation has fueled our success to date, but we believe that we have both the responsibility and potential to continue working for better and more reproducible outcomes. From planning to execution to postoperative data, intelligent surgery will proliferate our portfolios to provide better outcomes to the surgeon, patient and hospital.